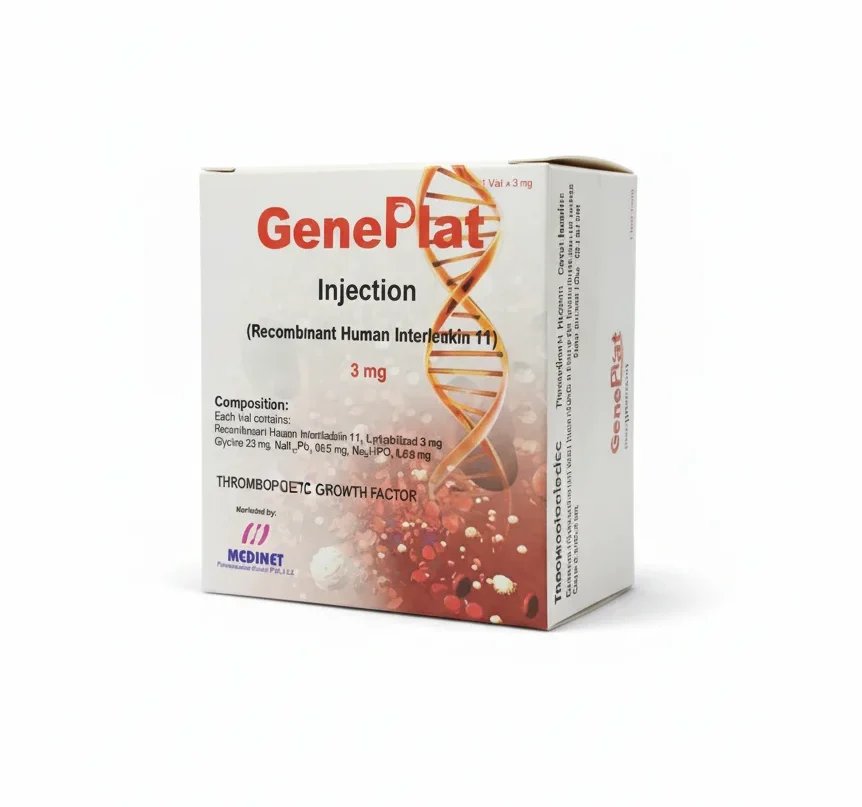
Geneplat 3mg Inj. 1`s
Geneplat Injection contains Oprelvekin, a recombinant human interleukin-11 (rhIL-11) used to prevent severe thrombocytopenia following myelosuppressive chemotherapy. Buy Geneplat 3mg injections at PakMeds for effective prevention of chemotherapy-induced thrombocytopenia.
| Manufacturer | Getz Pharma Pakistan |
| Active Ingredients | Oprelvekin (Recombinant Human Interleukin-11) |
| Medicine Strength | 3mg per vial |
| Formulation | Powder for Solution for Subcutaneous Injection |
| Requires Prescription? | Yes (Oncologist prescription required) |
| Generics | Oprelvekin, rhIL-11 |
Geneplat Ingredients and Usage
The active ingredient is Oprelvekin, a recombinant form of human interleukin-11 produced in Escherichia coli. It is administered as a daily subcutaneous injection, typically starting 6-24 hours after chemotherapy completion and continuing until platelet counts recover adequately.
Geneplat is clinically indicated for:
- Prevention of severe thrombocytopenia following myelosuppressive chemotherapy
- Reduction of platelet transfusion requirements in cancer patients
- Stimulation of megakaryocytopoiesis and thrombopoiesis
- Patients at high risk for chemotherapy-induced thrombocytopenia
How Does Geneplat Work?
Geneplat stimulates platelet production through hematopoietic growth factor activity:
- Megakaryocyte Stimulation: Promotes proliferation of hematopoietic stem cells and megakaryocyte progenitors
- Platelet Production: Increases megakaryocyte maturation and platelet production
- Hematopoietic Support: Supports overall hematopoiesis in the bone marrow
- Platelet Recovery: Accelerates platelet count recovery following chemotherapy
Geneplat Side Effects and Warnings
Common side effects include fluid retention, tachycardia, palpitations, dizziness, headache, and injection site reactions. Most adverse effects are reversible after discontinuation.
Serious Considerations
- Fluid Retention: May cause significant fluid retention leading to peripheral edema and dyspnea
- Cardiac Effects: Can cause tachycardia, atrial arrhythmias, and syncope
- Allergic Reactions: May cause hypersensitivity reactions including anaphylaxis
- Visual Disturbances: Can cause transient blurred vision and papilledema
- Renal Impairment: Use with caution in patients with pre-existing renal dysfunction
Geneplat Storage Conditions
Geneplat Injection requires strict storage conditions:
- Store unreconstituted vials at 2°C to 8°C
- Protect from light and freezing
- Use reconstituted solution within 3 hours
- Do not freeze reconstituted solution
- Discard unused portions appropriately