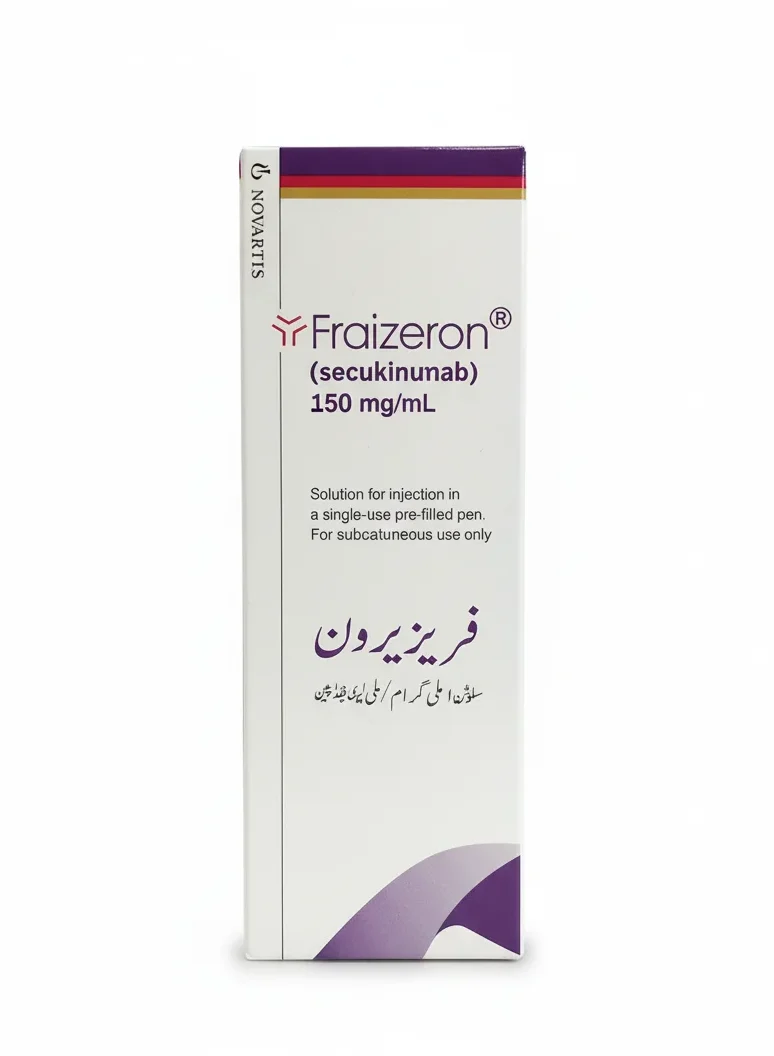
Fraizeron 150mg/ml Inj. 1`s
Fraizeron Injection contains Secukinumab, a human monoclonal antibody used for the treatment of moderate to severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis. Buy Fraizeron 150mg/ml injections at PakMeds for effective autoimmune disease management.
| Manufacturer | Novartis Pharma |
| Active Ingredients | Secukinumab |
| Medicine Strength | 150mg per ml (pre-filled syringe or vial) |
| Formulation | Solution for Subcutaneous Injection |
| Requires Prescription? | Yes (Dermatologist or Rheumatologist prescription required) |
| Generics | Secukinumab |
Fraizeron Ingredients and Usage
The active ingredient is Secukinumab, a fully human monoclonal IgG1/κ antibody that selectively binds to interleukin-17A. It is administered as subcutaneous injections with an initial loading dose followed by monthly maintenance doses.
Fraizeron is clinically indicated for:
- Moderate to severe plaque psoriasis in adults
- Active psoriatic arthritis in adults
- Ankylosing spondylitis (radiographic axial spondyloarthritis)
- Non-radiographic axial spondyloarthritis
How Does Fraizeron Work?
Fraizeron provides targeted immunomodulation through IL-17A inhibition:
- IL-17A Neutralization: Binds to and neutralizes interleukin-17A cytokine
- Inflammatory Pathway Blockade: Inhibits the release of pro-inflammatory cytokines
- Keratinocyte Regulation: Reduces keratinocyte proliferation and differentiation
- Immune Response Modulation: Modulates the Th17 immune response pathway
Fraizeron Side Effects and Warnings
Common side effects include upper respiratory infections, diarrhea, oral herpes, rhinitis, and injection site reactions. Most adverse effects are mild to moderate.
Serious Considerations
- Infections Risk: Increased susceptibility to infections including serious fungal and bacterial infections
- Inflammatory Bowel Disease: May cause new onset or exacerbation of inflammatory bowel disease
- Hypersensitivity Reactions: May cause serious allergic reactions including anaphylaxis
- Crohn’s Disease: Avoid use in patients with active Crohn’s disease
- Tuberculosis Screening: Evaluate for tuberculosis infection before initiating treatment
Fraizeron Storage Conditions
Fraizeron Injection requires strict storage conditions:
- Store at 2°C to 8°C in refrigerator
- Protect from light – keep in original carton
- Do not freeze or shake
- Allow to reach room temperature before injection (15-30 minutes)
- Do not use if discolored or contains particles