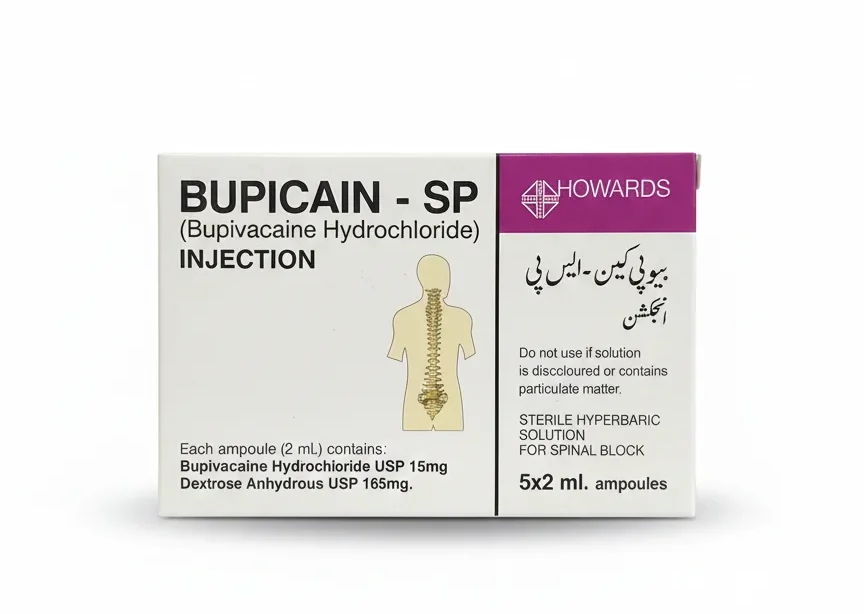
Bupicain SP Injection 15mg/2ml
Bupicain Spinal Injection contains Bupivacaine Hydrochloride, an amide-type local anesthetic renowned for its prolonged duration of action. Buy this essential anesthetic agent at PakMeds with proper specialist documentation.
| Manufacturer | Typically a local or regional pharmaceutical company (e.g., Sami, Getz Pharma, etc.) |
| Active Ingredients | Bupivacaine Hydrochloride (often with 8% Glucose for hyperbaric effect) |
| Common Strengths | 0.5% (i.e., 5 mg/mL) in a 4 mL ampoule or vial |
| Formulation | Hyperbaric Solution for Intrathecal (Spinal) Injection |
| Requires Prescription? | Yes (Anesthesiologist/Hospital use only) |
| Generics | Bupivacaine, Marcaine Spinal |
Bupicain Spinal 0.5% Ingredients and Usage
The active ingredient is Bupivacaine Hydrochloride. The addition of glucose makes the solution hyperbaric, meaning it sinks within the CSF, allowing the anesthesiologist to control the height of the block by adjusting the patient’s position.
Bupicain Spinal is clinically indicated for providing surgical anesthesia for procedures such as:
- Orthopedic Surgery: Hip, knee, and lower leg procedures.
- Urology/Gynecology: TURP (transurethral resection of the prostate), hysterectomy.
- General Surgery: Hernia repair, appendectomy.
- Cesarean Section (often with a lower concentration or isobaric formulation).
How Does Bupicain Spinal Work?
Bupivacaine is a voltage-gated sodium channel blocker. When injected into the CSF, it rapidly produces profound and reversible nerve block:
- Nerve Blockade: The drug diffuses into the nerve roots of the spinal cord and blocks the rapid influx of sodium ions (Na⁺) through the voltage-gated sodium channels.
- Action Potential Inhibition: By blocking Na⁺ influx, Bupivacaine prevents the generation and conduction of the nerve action potential.
- Differential Block: The result is a sequential loss of nerve function: first, pain sensation (sensory block) and temperature; followed by motor function (motor block). Bupivacaine is known for providing a long duration of both sensory and motor block (up to 3 to 4 hours).
- Hyperbaric Effect: The high density of the solution ensures that when injected in a specific position (e.g., sitting up), the drug moves predictably to the dependent areas of the spinal column.
Bupicain Spinal Side Effects and Warnings
Spinal anesthesia is safe, but administration requires immediate monitoring of vital signs due to the risk of hemodynamic changes and nerve complications.
Serious Warnings and Critical Precautions:
- Profound Hypotension and Bradycardia: Blockade of sympathetic nerves can cause a dramatic drop in blood pressure and heart rate. Immediate treatment with vasopressors (e.g., ephedrine or phenylephrine) is required.
- Total Spinal Anesthesia: Accidental upward spread of the anesthetic to the neck and head can cause respiratory depression, unconsciousness, and severe cardiovascular collapse, requiring immediate resuscitation.
- CNS Toxicity (Systemic Risk): While rare with spinal use, accidental rapid systemic absorption can cause central nervous system toxicity (e.g., seizures, perioral numbness, tinnitus) and cardiotoxicity (severe arrhythmias).
- Contraindications: Absolutely contraindicated in patients with septicemia, severe hypotension, coagulation disorders, or uncorrected hypovolemia. Should NEVER be used if the patient refuses the procedure.
- Aseptic Technique: Strict adherence to sterile technique is essential to prevent meningitis or other severe infections.
Bupicain Spinal Storage Conditions
Bupicain Spinal is a sterile solution requiring specific storage:
- Store the unopened ampoules/vials at controlled room temperature, typically between 20°C and 25°C (68°F and 77°F).
- Protect from Light: Keep the product in the original packaging until use.
- Inspection: The solution should be clear and colorless. Do not use if discolored or if particulate matter is present.
- Single Use: The formulation contains no preservative and is for single use only. Any unused portion must be discarded immediately.