No products in the cart.
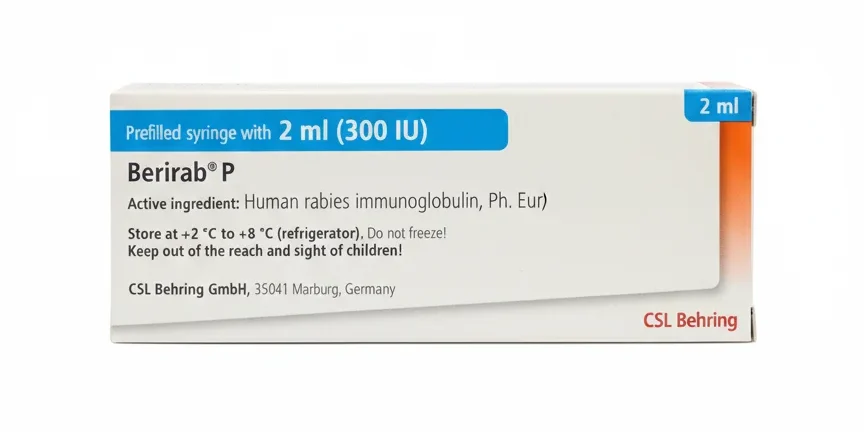
Berirab P Injection 300IU 2ml
Berirab P Injection contains Human Rabies Immunoglobulin (HRIG), a sterile solution of concentrated antibodies derived from human plasma donors. This is a high-alert, specialist-administered, prescription-only biological product.
₨ 12,560
7459
| Manufacturer | CSL Behring GmbH (Germany) |
| Active Ingredients | Human Rabies Immunoglobulin (HRIG) |
| Common Strengths | 300 IU/2 mL or 750 IU/5 mL (Varies by product and market) |
| Therapeutic Class | Passive Immunization Agent (Immunoglobulin) |
| Requires Prescription? | Yes (Emergency/Specialist use only) |
How Does Berirab P Work?
Berirab P is given as part of a two-pronged defense against rabies:
- Immediate Neutralization: HRIG contains pre-formed antibodies that immediately recognize and bind to the rabies virus particles at the site of exposure. This prevents the virus from entering the nerves and traveling to the brain.
- Passive Protection: This provides passive immunity—instant protection that lasts about 21 days. This covers the critical window before the patient’s own immune system can mount an active response from the simultaneously administered rabies vaccine.
Method of Administration
The total required dose of Berirab P is 20 IU per kg of body weight. The key to its efficacy is:
- Wound Infiltration: As much of the calculated dose as anatomically possible must be instilled deeply into and around the wound site(s) to neutralize the virus at the entry point.
- Intramuscular Injection: Any remaining portion is then injected into a muscle site (intramuscular, IM), preferably far from the wound.
- Timing: It should be given as soon as possible after exposure and always at the same time as, or up to 7 days after, the first dose of rabies vaccine.
Important Warnings and Precautions
- Administration Site: HRIG and the rabies vaccine must be given at separate anatomical sites (e.g., one in the thigh, one in the deltoid) to prevent the HRIG from neutralizing the vaccine’s active ingredients.
- Anaphylaxis Risk: As a blood product, there is a risk of severe allergic reactions (anaphylaxis). Patients must be monitored for at least 30 minutes after administration.
- Interference with Live Vaccines: HRIG can interfere with the development of immunity to other live attenuated viral vaccines (e.g., Measles, Mumps, Rubella). These vaccinations should generally be deferred for several months after HRIG administration.
- Storage: Berirab P is a cold-chain product. It must be stored in a refrigerator at 2°C to 8°C and MUST NOT BE FROZEN.