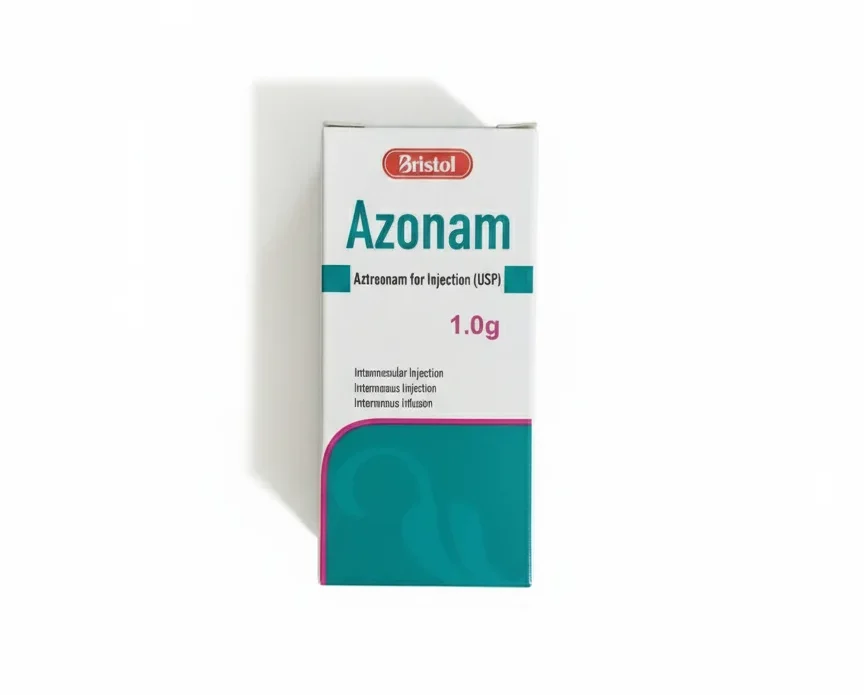
Azonam 1g Injection
Azonam 1 g Injection contains Aztreonam, a powerful, synthetic, monobactam antibiotic used in hospital settings to treat severe infections caused by susceptible aerobic Gram-negative bacteria. Procure this critical antibiotic at PakMeds with a valid prescription.
| Manufacturer | Typically a local or regional pharmaceutical company (e.g., Sami, Getz Pharma, etc.) |
| Active Ingredients | Aztreonam |
| Medicine Strength | 1 g per Vial (Lyophilized Powder) |
| Number Per Pack | Typically 1 Vial |
| Requires Prescription? | Yes (Specialist/Hospital use only) |
| Generics | Aztreonam |
Azonam 1 g Ingredients and Usage
The active ingredient is Aztreonam, a monobactam antibiotic. The 1 g vial is supplied as a lyophilized powder that must be reconstituted and then diluted for intravenous (IV) infusion (usually over 20 to 60 minutes) or for deep intramuscular (IM) injection.
Azonam is clinically indicated for the treatment of severe infections caused by susceptible Gram-negative organisms, including:
- Septicemia and Bacteremia (bloodstream infections).
- Lower Respiratory Tract Infections (e.g., pneumonia).
- Complicated Urinary Tract Infections (UTIs).
- Intra-abdominal Infections (e.g., peritonitis).
- Gynecologic Infections and serious Skin/Soft-Tissue Infections.
How Does Azonam Work?
Aztreonam is a bactericidal drug that rapidly kills susceptible bacteria. Its mechanism of action is characteristic of beta-lactam antibiotics, but its unique monocyclic structure grants it protection against many beta-lactamase enzymes and reduced cross-reactivity with penicillin/cephalosporin allergies:
- Cell Wall Interference: Aztreonam binds specifically to the penicillin-binding proteins (PBPs), particularly PBP3, which are enzymes essential for synthesizing the bacterial cell wall.
- Inhibition of Synthesis: By inhibiting these PBPs, Aztreonam prevents the cross-linking of peptidoglycan chains, leading to a defective and fragile bacterial cell wall.
- Lysis and Death: The weakened cell wall cannot withstand the internal osmotic pressure, causing the bacterial cell to rapidly swell, lyse (burst), and die.
Azonam 1 g Side Effects and Warnings
While generally well-tolerated, Aztreonam can cause side effects. Common side effects include pain/swelling at the injection site, rash, and diarrhea (potentially C. difficile-associated diarrhea).
Serious Warnings and Precautions:
- Hypersensitivity Reactions: Although the risk is low, serious, potentially fatal, hypersensitivity reactions (anaphylaxis) can occur, even in patients with penicillin allergies. Emergency resuscitation equipment must be readily available.
- Neutropenia/Thrombocytopenia: Hematological abnormalities, including a temporary reduction in white blood cells (neutropenia) and platelets (thrombocytopenia), may occur, requiring regular blood counts.
- Superinfection: As with all antibiotics, prolonged use can lead to the overgrowth of non-susceptible organisms, including fungi and Clostridium difficile, which can cause severe colitis (CDAD).
- Limited Gram-Positive Activity: Aztreonam has virtually no activity against Gram-positive bacteria or anaerobes; therefore, it is often used in combination with other antibiotics when treating polymicrobial infections.
Azonam 1 g Storage Conditions
Azonam 1 g is supplied as a powder requiring specific handling for IV administration:
- Store the unopened vials at controlled room temperature, typically below 25°C (77°F). Protect from excessive heat.
- Reconstitution: The powder must be reconstituted with a specific volume of Sterile Water for Injection, 0.9% Sodium Chloride, or other approved solutions.
- Dilution: The reconstituted solution must then be further diluted in a suitable IV fluid (e.g., 0.9% NaCl or 5% Dextrose) before slow infusion.
- Stability: The stability of the reconstituted and diluted solution depends on the diluent used and the temperature, often requiring use within 24 to 48 hours.