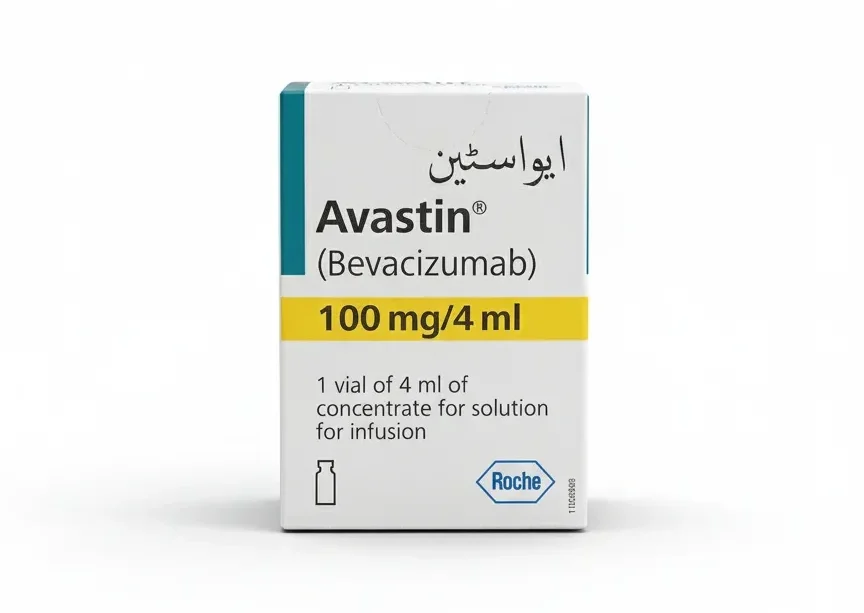
Avastin 100mg/ 4ml Injection
Avastin 100 mg/4 mL (Bevacizumab) is a specialized, targeted biological therapy used to treat several advanced or metastatic cancers, including colorectal, lung, kidney, cervical, and ovarian cancers, as well as glioblastoma. Secure this vital cancer treatment at PakMeds with a valid specialist prescription.
| Manufacturer | Roche (Genentech/Novartis globally) / Local authorized distributor |
| Active Ingredients | Bevacizumab |
| Medicine Strength | 25 mg/mL (100 mg total in 4 mL vial) |
| Number Per Pack | 1 Single-Use Vial (4 mL) |
| Requires Prescription? | Yes (Oncologist/Specialist Prescription) |
| Generics | Bevacizumab (Biosimilars available) |
Avastin 100 mg/4 mL Ingredients and Usage
The active ingredient is Bevacizumab, a recombinant humanized monoclonal antibody. It is supplied as a sterile, preservative-free concentrate that must be diluted before being administered as an intravenous (IV) infusion.
Avastin is clinically indicated for use in combination with standard chemotherapy for treating:
- Metastatic Colorectal Cancer (mCRC)
- Non-Small Cell Lung Cancer (NSCLC)
- Metastatic Renal Cell Carcinoma (mRCC) (Kidney Cancer)
- Glioblastoma (Brain Tumor)
- Ovarian Cancer and Cervical Cancer (in advanced stages)
How Does Avastin Work?
Avastin works by targeting the crucial process of angiogenesis, the formation of new blood vessels needed to feed tumor growth. Its mechanism is highly specific:
- VEGF Binding: Bevacizumab binds specifically and tightly to Vascular Endothelial Growth Factor (VEGF), a protein messenger essential for stimulating new blood vessel growth.
- VEGF Neutralization: By binding to VEGF, Avastin prevents it from attaching to its receptors (VEGFR) on the surface of endothelial cells (the cells lining blood vessels).
- Anti-Angiogenesis: This neutralization starves the tumor by inhibiting the formation of new blood vessels and causing the regression of existing, immature tumor vessels. This slows tumor growth, limits metastasis, and can also improve the delivery of co-administered chemotherapy.
Avastin 100 mg/4 mL Side Effects and Warnings
Avastin is a high-alert medication with several serious associated risks. Patients must be closely monitored, especially for signs of bleeding and perforation. Common side effects include hypertension (high blood pressure), fatigue, diarrhea, and pain.
Black Box Warnings and Critical Precautions:
- Gastrointestinal Perforation: This is a severe, potentially fatal risk. Avastin can cause perforations (holes) in the stomach, intestines, or rectum. This risk is higher in patients with a history of digestive tract disease.
- Hemorrhage (Bleeding): Significant, sometimes fatal, bleeding events can occur, including GI bleeding, intracranial hemorrhage (bleeding in the brain), and pulmonary hemorrhage (bleeding in the lungs). Avastin must be discontinued in patients who experience a severe bleeding event.
- Thromboembolism: Increased risk of developing arterial thromboembolic events (ATE), such as stroke and heart attack, especially in patients with a history of these events.
- Wound Healing Complications: Avastin can impair wound healing. It is typically withheld for 28 days before and after major surgery until the surgical wound is fully healed.
- Proteinuria: Development of significant protein in the urine, sometimes progressing to nephrotic syndrome, requires regular urine monitoring.
Avastin 100 mg/4 mL Storage Conditions
Avastin is a highly sensitive biological product requiring strict cold storage protocols:
- Store the unopened vial in a refrigerator at a temperature between 2°C and 8°C (36°F and 46°F).
- DO NOT FREEZE. Freezing can permanently damage the protein structure, rendering the drug inactive.
- Protect from Light: Keep the vial in the original carton to protect the solution from light.
- Dilution and Stability: The concentrate must be diluted in 0.9% Sodium Chloride solution. The diluted solution is generally stable for up to 24 hours at 2°C to 30°C but should ideally be infused immediately.