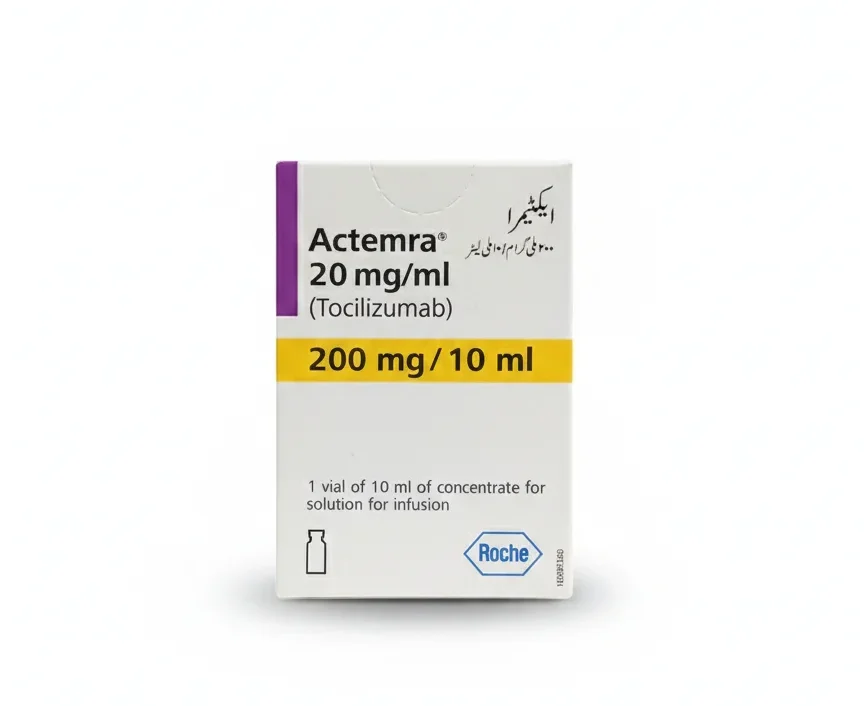
Actemra 200mg / 10ml
Actemra 200 mg/10 mL (Tocilizumab) is a targeted biological therapy used to treat several severe inflammatory and autoimmune diseases, including Rheumatoid Arthritis (RA), Systemic Juvenile Idiopathic Arthritis (SJIA), Polyarticular Juvenile Idiopathic Arthritis (PJIA), and Giant Cell Arteritis (GCA). Obtain this prescription-only biologic therapy at PakMeds with the required documentation.
| Manufacturer | F. Hoffmann-La Roche Ltd. / Roche |
| Active Ingredient | Tocilizumab |
| Strength | 200 mg in 10 mL (Concentrate for Solution for Infusion) |
| Pack Size | 1 Single-Use Vial |
| Requires Prescription? | Yes (Specialist / Biologic Therapy Prescription) |
| Generic/Biosimilars | Tocilizumab (biosimilars may be available) |
Actemra 200 mg/10 mL – Ingredients and Usage
The active ingredient in Actemra is Tocilizumab, a humanized recombinant monoclonal antibody that targets the IL-6 receptor. It is supplied as a sterile, preservative-free concentrate that must be diluted prior to intravenous infusion.
Actemra is indicated for the treatment of:
- Moderate to severe active Rheumatoid Arthritis (RA) in adults who have had an inadequate response to DMARDs or TNF inhibitors
- Systemic Juvenile Idiopathic Arthritis (SJIA) in children aged 2 years and older
- Polyarticular Juvenile Idiopathic Arthritis (PJIA) in children aged 2 years and older
- Giant Cell Arteritis (GCA) in adults
- Severe COVID-19 in hospitalized adults receiving corticosteroids and requiring supplemental oxygen or ventilation (depending on region-specific approval or emergency use)
How Actemra Works
Actemra reduces inflammation by blocking the activity of Interleukin-6 (IL-6), one of the major cytokines involved in autoimmune diseases.
- IL-6 Receptor Binding: Tocilizumab binds to both soluble and membrane-bound IL-6 receptors (IL-6R).
- Signal Inhibition: This prevents IL-6 from triggering intracellular signaling pathways that promote inflammation.
- Reduction of Symptoms: By suppressing IL-6 activity, Actemra decreases joint swelling, pain, fatigue, systemic inflammation, and helps prevent long-term joint damage.
Actemra 200 mg/10 mL – Side Effects and Warnings
Because Actemra suppresses immune function, it may increase susceptibility to infections. Common side effects include upper respiratory tract infections, headache, and hypertension. Liver enzyme elevations and changes in blood counts (neutropenia, thrombocytopenia) may also occur, requiring routine monitoring.
Serious Warnings and Precautions:
- Serious Infections: Increased risk of severe bacterial, viral, or fungal infections, including tuberculosis (TB). Patients must be screened for latent TB before therapy. Treatment should be delayed in cases of active infection.
- Gastrointestinal Perforation: Particularly in patients with diverticulitis or history of GI ulcers. New abdominal pain should be reported immediately.
- Hypersensitivity and Anaphylaxis: Serious infusion-related hypersensitivity reactions may occur. Infusions must be administered in a facility with emergency support.
- Vaccinations: Live vaccines should not be administered concurrently with Tocilizumab due to reduced vaccine efficacy and increased infection risk.
Actemra 200 mg/10 mL – Storage Conditions
- Store unopened vials in a refrigerator at 2°C to 8°C (36°F to 46°F).
- Do not freeze. Freezing can damage the biological product.
- Protect vials from light by keeping them in their original carton until use.
- Once diluted, the solution should be used as soon as possible. Stability is typically maintained for up to 24 hours when refrigerated (2°C to 8°C) or for 4 hours at room temperature, depending on the clinical protocol.
- Keep out of reach of children.